Kelly Warner Law Firm Blames USA Herald for Arizona Bar Investigation
Mr. Levy publishes this about his filing, noting that the Kelly Warner Law Firm was “Defrauding an Arizona Court” with…
By – USA HeraldAaron Kelly Law Firm Resorts To Attacking Former Client Again On KellyWarnerLaw.com – Pattern Recognized
The irony here is that Dan Warner was accused of publishing a defamatory post on KellyWarnerLaw.com on his former client…
By – Jeff WattersonArizona Bar Opens Investigation on Attorney Aaron Kelly
Volokh, as part of his investigative work, had a private investigator research the defendants in some of theallegedly fraudulent…
By – Paul O'NealBritney Spears Arrested for DUI in California After Early Morning Traffic Stop
Touring Success and Uncertain Future Performances Spears’ last major tour came in 2018, when she launched the Piece of Me…
By – Rachel MooreUS Draft AI Chip Sales Rules Could Reshape Global Tech Power Balance
Global Monitoring of Chip Flows One consequence of the US draft AI chip sales rules could be significantly improved oversight…
By – Rachel MooreSix Flags Amusement Parks Sale Signals Major Shake-Up in Theme Park Industry
Revenue Pressures Drive Strategy Changes At the time, then-CEO Richard Zimmerman acknowledged that the company’s efforts to boost attendance had…
By – Rachel MooreVast $500M Funding Fuels Race to Build the Next Generation of Space Stations
More Than $1 Billion Invested in Space Infrastructure With the latest funding, total investment in Vast’s space station technologies and…
By – Rihem AkkoucheAlexander Brothers Sex-Trafficking Trial Heads to Jury After Dramatic Closing Arguments
New Lawsuit Emerges During Trial Even as the criminal trial moved toward a verdict, the legal troubles surrounding the brothers…
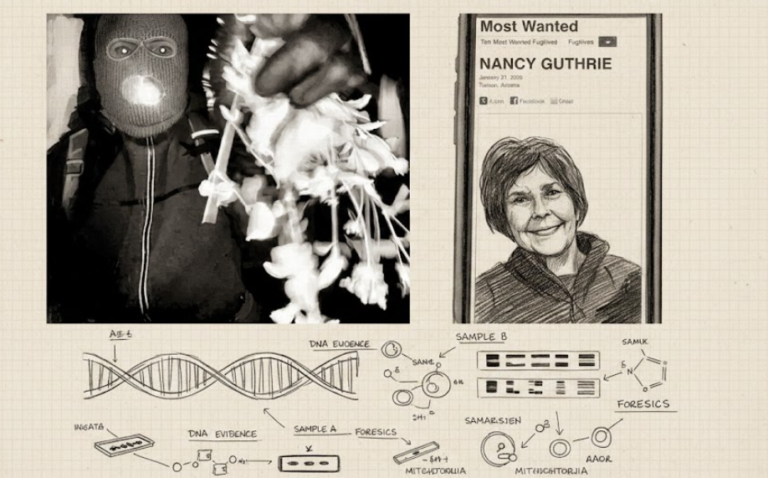
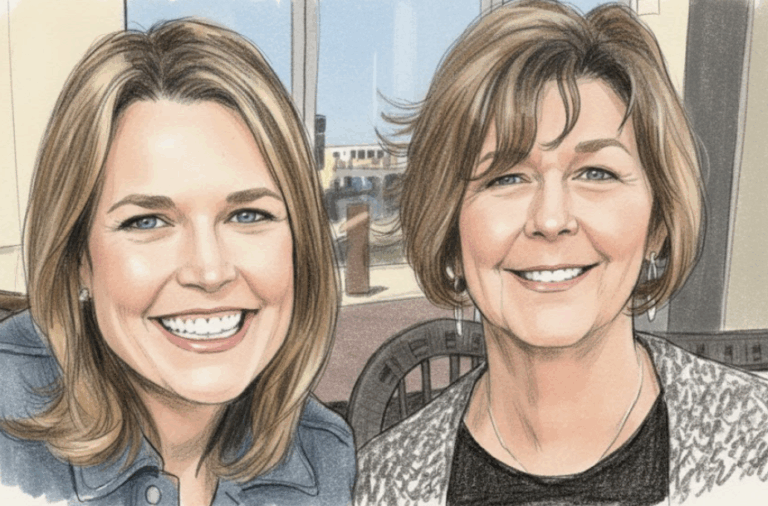
By – Rihem AkkoucheSavannah Guthrie Return to NBC Planned Amid Ongoing Search for Missing Mother
Evidence Found — But No Match In another twist, investigators recovered DNA evidence from a glove found near Nancy Guthrie’s…
By – Rihem AkkoucheBritney Spears Arrested for DUI in California After Early Morning Traffic Stop
Touring Success and Uncertain Future Performances Spears’ last major tour came in 2018, when she launched the Piece of Me…
By – Rachel MooreUS Draft AI Chip Sales Rules Could Reshape Global Tech Power Balance
Global Monitoring of Chip Flows One consequence of the US draft AI chip sales rules could be significantly improved oversight…
By – Rachel MooreSix Flags Amusement Parks Sale Signals Major Shake-Up in Theme Park Industry
Revenue Pressures Drive Strategy Changes At the time, then-CEO Richard Zimmerman acknowledged that the company’s efforts to boost attendance had…
By – Rachel MooreVast $500M Funding Fuels Race to Build the Next Generation of Space Stations
More Than $1 Billion Invested in Space Infrastructure With the latest funding, total investment in Vast’s space station technologies and…
By – Rihem AkkoucheAlexander Brothers Sex-Trafficking Trial Heads to Jury After Dramatic Closing Arguments
New Lawsuit Emerges During Trial Even as the criminal trial moved toward a verdict, the legal troubles surrounding the brothers…
By – Rihem AkkoucheSavannah Guthrie Return to NBC Planned Amid Ongoing Search for Missing Mother
Evidence Found — But No Match In another twist, investigators recovered DNA evidence from a glove found near Nancy Guthrie’s…
By – Rihem AkkoucheBritney Spears Arrested for DUI in California After Early Morning Traffic Stop
Touring Success and Uncertain Future Performances Spears’ last major tour came in 2018, when she launched the Piece of Me…
By – Rachel MooreUS Draft AI Chip Sales Rules Could Reshape Global Tech Power Balance
Global Monitoring of Chip Flows One consequence of the US draft AI chip sales rules could be significantly improved oversight…
By – Rachel MooreSix Flags Amusement Parks Sale Signals Major Shake-Up in Theme Park Industry
Revenue Pressures Drive Strategy Changes At the time, then-CEO Richard Zimmerman acknowledged that the company’s efforts to boost attendance had…
By – Rachel MooreVast $500M Funding Fuels Race to Build the Next Generation of Space Stations
More Than $1 Billion Invested in Space Infrastructure With the latest funding, total investment in Vast’s space station technologies and…
By – Rihem AkkoucheAlexander Brothers Sex-Trafficking Trial Heads to Jury After Dramatic Closing Arguments
New Lawsuit Emerges During Trial Even as the criminal trial moved toward a verdict, the legal troubles surrounding the brothers…
By – Rihem AkkoucheSavannah Guthrie Return to NBC Planned Amid Ongoing Search for Missing Mother
Evidence Found — But No Match In another twist, investigators recovered DNA evidence from a glove found near Nancy Guthrie’s…
By – Rihem AkkoucheNASA Pushes Lunar Return to 2028 Amid Artemis Program Timeline Revision
The revised timeline represents a balance between ambition and technical realism. While delays are common in complex human spaceflight programs,…
By – Ahmed BoughallebU.S. And Israel Launch Major Strikes On Iran — What It Means For America
Continuation of military strikes if Iran persists in retaliatory attacks. Diplomacy or ceasefire negotiations if international pressure increases. A broader…
By – Samuel LopezU.S. Court of Appeals for the Ninth Circuit Overturns $8M Asbestos Verdict Against BNSF Railway Co.
BNSF declined to comment on the decision. Judges Consuelo M. Callahan, Morgan B. Christen and Andrew D. Hurwitz sat on…
By – Tyler BrooksMissing DNA Evidence May Limit Investigation Into Nancy Guthrie Case, Sources Suggest
Grace Samuelson and Alex Sundby contributed reporting.
By – Ahmed BoughallebGrand Theft Auto 6 Targets November 2026 Launch as Price Leak Fuels $100 Debate
If early reports prove accurate, GTA 6 could redefine expectations for open-world games — both in scope and cost. Whether…
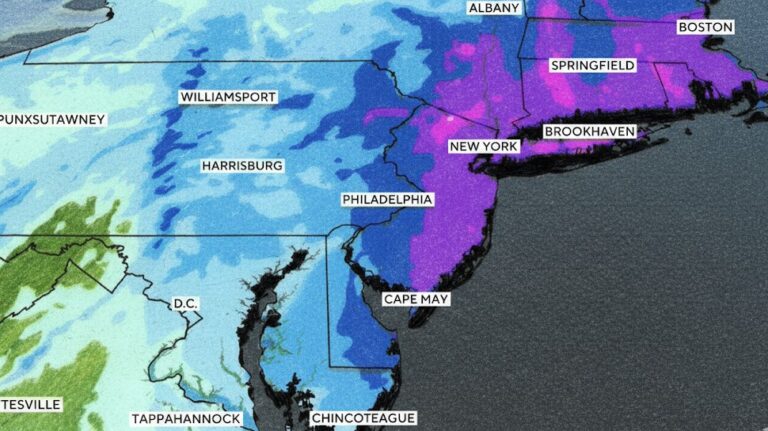
By – Ahmed Boughalleb40 Million Under Blizzard Warnings as Major Winter Storm Threatens U.S. East Coast
Officials continue urging residents to stay at home if possible, prepare emergency supplies, and follow local weather alerts until the…
By – Tyler BrooksBritney Spears Arrested for DUI in California After Early Morning Traffic Stop
Touring Success and Uncertain Future Performances Spears’ last major tour came in 2018, when she launched the Piece of Me…
By – Rachel MooreUS Draft AI Chip Sales Rules Could Reshape Global Tech Power Balance
Global Monitoring of Chip Flows One consequence of the US draft AI chip sales rules could be significantly improved oversight…
By – Rachel MooreSix Flags Amusement Parks Sale Signals Major Shake-Up in Theme Park Industry
Revenue Pressures Drive Strategy Changes At the time, then-CEO Richard Zimmerman acknowledged that the company’s efforts to boost attendance had…
By – Rachel MooreVast $500M Funding Fuels Race to Build the Next Generation of Space Stations
More Than $1 Billion Invested in Space Infrastructure With the latest funding, total investment in Vast’s space station technologies and…
By – Rihem AkkoucheAlexander Brothers Sex-Trafficking Trial Heads to Jury After Dramatic Closing Arguments
New Lawsuit Emerges During Trial Even as the criminal trial moved toward a verdict, the legal troubles surrounding the brothers…
By – Rihem AkkoucheSavannah Guthrie Return to NBC Planned Amid Ongoing Search for Missing Mother
Evidence Found — But No Match In another twist, investigators recovered DNA evidence from a glove found near Nancy Guthrie’s…
By – Rihem AkkoucheFDA Announces Recall of Rhino Choco VIP 10X Supplement After Undeclared Erectile Dysfunction Drug Found in Chocolate Product
The investigation into the recalled chocolate supplement remains ongoing.
By – Ahmed BoughallebWhen The Files Are Finally Unsealed The Most Mind-Bending Truth May Not Be What We Expect
[USA HERALD] – There is a widespread assumption that if governments release their most highly classified files related to unidentified…
By – Samuel LopezCivil Rights Icon Rev. Jesse Jackson Dies at 84 As President Trump Issues Personal Tribute
His work with the Rainbow PUSH Coalition institutionalized activism in a way that outlived any single protest or election cycle.…
By – Samuel LopezClues to Savannah Guthrie Missing Mom’s Disappearance Found on Security System
Savannah Guthrie Concerned about Ongoing Investigation With the case entering its second week, investigators remain focused on integrating physical evidence…
By – Jackie AllenMike Tyson Urges Americans to ‘Eat Real Food’ in Emotional Super Bowl Ad Highlighting Health Risks
Boxing legend Mike Tyson is using his platform ahead of Super Bowl 60 to address a personal and national health…
By – Tyler BrooksDeadly “Death Cap” Mushrooms in California Cause Multiple Deaths and Liver Transplants Amid Rare Super Bloom
California health officials are warning the public after four deaths and three liver transplants linked to the highly toxic death…
By – Ahmed BoughallebCadillac Names Inaugural Formula 1 Car MAC-26 in Tribute to Mario Andretti Ahead of 2026 Australian Grand Prix Debut
However, the combination of experienced drivers, established engine supply and corporate backing provides reasons for cautious optimism. The 2026 regulations…
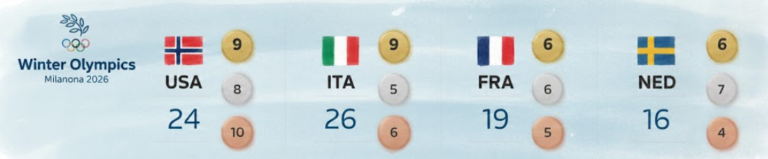
By – Ahmed BoughallebNorway Tops Medal Table After Day 13 at 2026 Winter Olympics as Team USA Surges Into Second Place
Upcoming Medal Events Medal competition continues Friday, February 20, with several key finals scheduled. Events awarding medals include: Women’s ski…
By – Ahmed BoughallebOlympic Science Explained: How Figure Skaters Spin at Blinding Speeds Without Getting Dizzy
By – Tyler Brooks
Olympic Villages Run Out of Condoms at 2026 Milan-Cortina Games
Condom supplies in the Olympic Villages at the 2026 Winter Games have been temporarily depleted, the Milan-Cortina organizing committee confirmed,…
By – Tyler BrooksArizona Authorities Escalate Search for Savannah Guthrie’s Mom to a Criminal Investigation
USA TODAY’s live coverage: https://www.usatoday.com Pima County Sheriff’s Office: https://www.pimasheriff.org NBC News reporting: https://www.nbcnews.com As the investigation continues, officials say…
By – Jackie AllenWhat is Aegosexuality?
Take berrisexual, for example. Urban Dictionary describes it as attraction primarily to women, feminine-aligned genders, and androgynous individuals, with only…
By – Jackie AllenNo posts found.
No posts found.
 No comments yet. Be the first to comment!
No comments yet. Be the first to comment!