Kelly Warner Law Firm Blames USA Herald for Arizona Bar Investigation
In what appears as a desperate attempt to defend multiple allegations of fraud on the courts, the Kelly Warner Law…
By – USA HeraldAaron Kelly Law Firm Resorts To Attacking Former Client Again On KellyWarnerLaw.com – Pattern Recognized
Attorney Aaron Kelly and his law partner Daniel Warner are currently under investigation by the Arizona Bar for legal misconduct.…
By – Jeff WattersonArizona Bar Opens Investigation on Attorney Aaron Kelly
USA Herald recently reported on a developing story involving Attorneys Daniel Warner and Aaron Kelly. Both Warner and Kelly have…
By – Paul O'NealStrike on Iranian Primary School Investigated as War Crime, According to HRW
On a Saturday morning in late February, as hundreds of missiles and guided bombs rained down across Iran, one struck…
By – Rochdi RaisIPIC Theaters Files for Bankruptcy as Luxury Cinema Chain Seeks Lifeline
The phrase IPIC Theaters files for bankruptcy now marks the latest twist in the turbulent story of America’s movie theater…
By – Rachel MooreUS Cuba Economic Deal Emerges as Trump Administration Eyes Major Policy Shift
A potential US Cuba Economic Deal may soon reshape relations between Washington and Havana, according to two sources familiar with…
By – Rachel MooreAmazon FCC Denial of SpaceX Plan Urged in Satellite Showdown
A fierce new battle is unfolding in the race to dominate space-based internet as Amazon FCC denial of SpaceX plan…
By – Rihem AkkoucheCountry Joe McDonald Died at 84, Woodstock Legend and Protest Voice Falls Silent
The music world is mourning as Country Joe McDonald died at the age of 84, closing the chapter on a…
By – Rihem AkkoucheThe Northern Lights Return
The Northern Lights have a chance to be visible from several northern U.S. states on Tuesday night, forecasters at the…
By – Jackie AllenStrike on Iranian Primary School Investigated as War Crime, According to HRW
On a Saturday morning in late February, as hundreds of missiles and guided bombs rained down across Iran, one struck…
By – Rochdi RaisIPIC Theaters Files for Bankruptcy as Luxury Cinema Chain Seeks Lifeline
The phrase IPIC Theaters files for bankruptcy now marks the latest twist in the turbulent story of America’s movie theater…
By – Rachel MooreUS Cuba Economic Deal Emerges as Trump Administration Eyes Major Policy Shift
A potential US Cuba Economic Deal may soon reshape relations between Washington and Havana, according to two sources familiar with…
By – Rachel MooreAmazon FCC Denial of SpaceX Plan Urged in Satellite Showdown
A fierce new battle is unfolding in the race to dominate space-based internet as Amazon FCC denial of SpaceX plan…
By – Rihem AkkoucheCountry Joe McDonald Died at 84, Woodstock Legend and Protest Voice Falls Silent
The music world is mourning as Country Joe McDonald died at the age of 84, closing the chapter on a…
By – Rihem AkkoucheThe Northern Lights Return
The Northern Lights have a chance to be visible from several northern U.S. states on Tuesday night, forecasters at the…
By – Jackie AllenIPIC Theaters Files for Bankruptcy as Luxury Cinema Chain Seeks Lifeline
The phrase IPIC Theaters files for bankruptcy now marks the latest twist in the turbulent story of America’s movie theater…
By – Rachel MooreUS Cuba Economic Deal Emerges as Trump Administration Eyes Major Policy Shift
A potential US Cuba Economic Deal may soon reshape relations between Washington and Havana, according to two sources familiar with…
By – Rachel MooreAmazon FCC Denial of SpaceX Plan Urged in Satellite Showdown
A fierce new battle is unfolding in the race to dominate space-based internet as Amazon FCC denial of SpaceX plan…
By – Rihem AkkoucheCountry Joe McDonald Died at 84, Woodstock Legend and Protest Voice Falls Silent
The music world is mourning as Country Joe McDonald died at the age of 84, closing the chapter on a…
By – Rihem AkkoucheThe Northern Lights Return
The Northern Lights have a chance to be visible from several northern U.S. states on Tuesday night, forecasters at the…
By – Jackie AllenMalaysia Airlines MH370 Disappearance Remains Unsolved After New Ocean Search
More than a decade after one of aviation’s greatest mysteries began, the Malaysia Airlines MH370 disappearance remains unsolved as a…
By – Rihem AkkoucheThe Northern Lights Return
The Northern Lights have a chance to be visible from several northern U.S. states on Tuesday night, forecasters at the…
By – Jackie AllenFebruary Unemployment Up as Job Losses Surprise Economists
February Unemployment Up as the latest labor market data revealed weaker-than-expected job growth and a slight increase in the national…
By – Jackie AllenLate-Night Attack by Venezuelan National at Florida Beach
A Late-night attack by a Venezuelan National has left a Florida community shaken after authorities say a 26-year-old man ambushed…
By – Jackie AllenTrump’s War in Iran: Congress Confronts Escalation After U.S. Strikes
Trump’s War in Iran was triggered open conflict, casualties, and renewed constitutional debate in Washington. The crisis intensified following reports…
By – Jackie AllenU.S. And Israel Launch Major Strikes On Iran — What It Means For America
TEHRAN, Iran – In a dramatic escalation of global tensions, the United States and Israel launched coordinated military strikes against Iran…
By – Samuel LopezU.S. Court of Appeals for the Ninth Circuit Overturns $8M Asbestos Verdict Against BNSF Railway Co.
The U.S. Court of Appeals for the Ninth Circuit has thrown out an $8 million jury verdict against BNSF Railway…
By – Tyler BrooksIPIC Theaters Files for Bankruptcy as Luxury Cinema Chain Seeks Lifeline
The phrase IPIC Theaters files for bankruptcy now marks the latest twist in the turbulent story of America’s movie theater…
By – Rachel MooreUS Cuba Economic Deal Emerges as Trump Administration Eyes Major Policy Shift
A potential US Cuba Economic Deal may soon reshape relations between Washington and Havana, according to two sources familiar with…
By – Rachel MooreAmazon FCC Denial of SpaceX Plan Urged in Satellite Showdown
A fierce new battle is unfolding in the race to dominate space-based internet as Amazon FCC denial of SpaceX plan…
By – Rihem AkkoucheCountry Joe McDonald Died at 84, Woodstock Legend and Protest Voice Falls Silent
The music world is mourning as Country Joe McDonald died at the age of 84, closing the chapter on a…
By – Rihem AkkoucheThe Northern Lights Return
The Northern Lights have a chance to be visible from several northern U.S. states on Tuesday night, forecasters at the…
By – Jackie AllenMalaysia Airlines MH370 Disappearance Remains Unsolved After New Ocean Search
More than a decade after one of aviation’s greatest mysteries began, the Malaysia Airlines MH370 disappearance remains unsolved as a…
By – Rihem AkkoucheWhen The Files Are Finally Unsealed The Most Mind-Bending Truth May Not Be What We Expect
[USA HERALD] – There is a widespread assumption that if governments release their most highly classified files related to unidentified…
By – Samuel LopezCivil Rights Icon Rev. Jesse Jackson Dies at 84 As President Trump Issues Personal Tribute
[USA HERALD] — The Rev. Jesse Jackson, a towering figure of the American civil rights movement whose career spanned more than…
By – Samuel LopezClues to Savannah Guthrie Missing Mom’s Disappearance Found on Security System
The disappearance of the mother of Savannah Guthrie has taken a dramatic turn as investigators focus on digital clues tied…
By – Jackie AllenMike Tyson Urges Americans to ‘Eat Real Food’ in Emotional Super Bowl Ad Highlighting Health Risks
Boxing legend Mike Tyson is using his platform ahead of Super Bowl 60 to address a personal and national health…
By – Tyler BrooksDeadly “Death Cap” Mushrooms in California Cause Multiple Deaths and Liver Transplants Amid Rare Super Bloom
California health officials are warning the public after four deaths and three liver transplants linked to the highly toxic death…
By – Ahmed BoughallebFrom Migraines to Miracles: How Becca Valle Survived a Glioblastoma Diagnosis Against the Odds
Becca Valle, 41, thought her headaches were just migraines—until a sudden, unbearable pain revealed something far more serious. In September…
By – Tyler BrooksFebruary Unemployment Up as Job Losses Surprise Economists
February Unemployment Up as the latest labor market data revealed weaker-than-expected job growth and a slight increase in the national…
By – Jackie AllenLate-Night Attack by Venezuelan National at Florida Beach
A Late-night attack by a Venezuelan National has left a Florida community shaken after authorities say a 26-year-old man ambushed…
By – Jackie AllenTrump’s War in Iran: Congress Confronts Escalation After U.S. Strikes
Trump’s War in Iran was triggered open conflict, casualties, and renewed constitutional debate in Washington. The crisis intensified following reports…
By – Jackie AllenCadillac Names Inaugural Formula 1 Car MAC-26 in Tribute to Mario Andretti Ahead of 2026 Australian Grand Prix Debut
Cadillac has officially revealed the name of its first Formula 1 challenger, confirming that its 2026 car will be called…
By – Ahmed BoughallebNorway Tops Medal Table After Day 13 at 2026 Winter Olympics as Team USA Surges Into Second Place
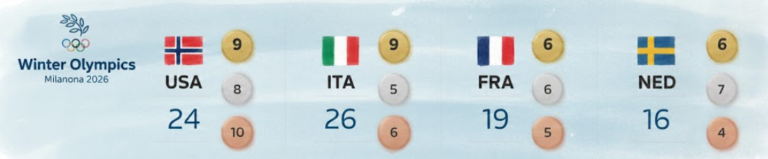
With 13 days complete at the 2026 Milan Cortina Winter Olympics, Norway sits atop the overall medal standings, collecting 34…
By – Ahmed BoughallebOlympic Science Explained: How Figure Skaters Spin at Blinding Speeds Without Getting Dizzy
When Amber Glenn finishes her routine, the arena usually rises with her. The music builds, her blades carve a tight…
By – Tyler BrooksNo posts found.
No posts found.
 No comments yet. Be the first to comment!
No comments yet. Be the first to comment!